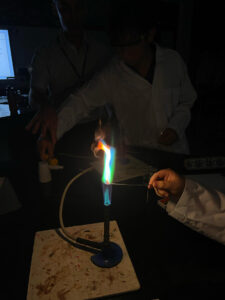
Today, the students were taught how to perform a flame test – an analytical technique used to identify the presence of certain metal ions in a sample based on the distinct colors they produce when heated in a flame. They carried out the flame test on five different metal salts containing the following ions: Lithium, Sodium, Potassium, Copper and Zinc. Through this practical, they also learned the importance of avoiding contamination between the salts as to obtain accurate results.
The second portion of the lab was based around the precipitate tests, a process used to identify the presence of specific ions in a solution based on the colour of the insoluble compounds formed. They learned to distinguish between the Iron (II), Iron (III) and Copper (II) ions.